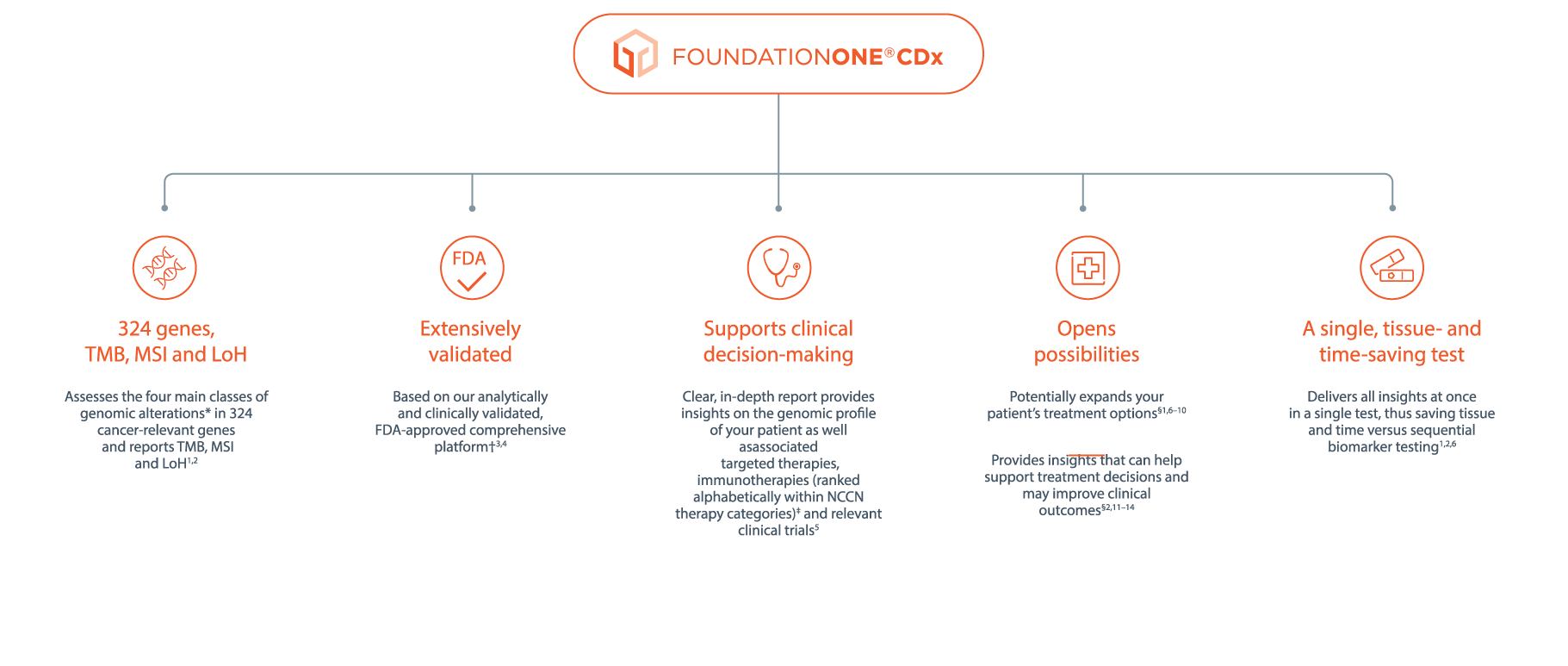
*Base substitutions, insertions or deletions, copy number alterations and gene rearrangements.
†Clinical validation demonstrated concordance with the following companion diagnostics: cobas® EGFR Mutation Test, Ventana ALK (D5F3) CDx Assay, Vysis ALK Break-Apart FISH Probe Kit, therascreen® KRAS RGQ PCR Kit, Dako HER2 FISH PharmDx® Kit, cobas® BRAF V600 Mutation Test, THxID® BRAF kit. For more information, please see the FoundationOne®CDx Technical Information available at: www.rochefoundationmedicine.com/f1cdxtech.
‡For additional information on the NCCN categories please refer to the NCCN Compendium® (www.nccn.org).
§Based on a concordance study with FoundationOne®. FoundationOne CDx leverages the same comprehensive genomic profiling approach and is highly concordant with FoundationOne.
EGFR, epidermal growth factor receptor; FDA, US Food and Drug Administration; FISH, fluorescence in situ hybridisation; LoH, Loss of Heterozygosity; IHC, immunohistochemistry; MSI, Microsatellite Instability; NCCN, National Comprehensive Cancer Network; NGS, next generation sequencing; NSCLC, non-small cell lung cancer; PARP, poly-ADP ribose polymerase; PD-L1, programmed cell death ligand 1; TKI, tyrosine kinase inhibitor; TMB, Tumour Mutational Burden.